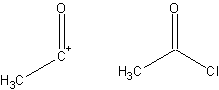|
E. J. Corey
Elias James Corey (born July 12, 1928) is an American organic chemistry, organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many as one of the greatest living chemists, he has developed numerous synthetic reagents, methodologies and total syntheses and has advanced the science of organic synthesis considerably. Biography E.J. Corey (the surname was anglicized from Levantine Arabic ''Khoury'', meaning ''priest'') was born to Lebanese Greek Orthodox Christians, Lebanese Greek Orthodox Christian immigrants Fatima (née Hasham) and Elias Corey in Methuen, Massachusetts, north of Boston. His mother changed his name from William to "Elias" to honor his father, who died eighteen months after Corey's birth. His widowed mother, brother, two sisters, aunt and uncle all lived together in a spacious house, struggling through the Great Depression. As a young ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methuen, Massachusetts
Methuen () is a 23-square-mile (60 km2) city in Essex County, Massachusetts, United States. The population was 53,059 at the 2020 United States census, 2020 census. Methuen lies along the northwestern edge of Essex County, just east of Middlesex County, Massachusetts, Middlesex County and just south of Rockingham County, New Hampshire. The city is bordered by Haverhill, Massachusetts, Haverhill to the northeast, North Andover, Massachusetts, North Andover to the southeast, Lawrence, Massachusetts, Lawrence and Andover, Massachusetts, Andover to the south, Dracut, Massachusetts, Dracut (Middlesex County) to the west, Pelham, New Hampshire (Hillsborough County, New Hampshire, Hillsborough County) to the northwest, and Salem, New Hampshire (Rockingham County, New Hampshire, Rockingham County) to the north. Methuen is located southwest from Newburyport, north-northwest of Boston and south-southeast of Manchester, New Hampshire. The city is a part of the Merrimack Valley and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ryōji Noyori
is a Japanese chemist. He won the Nobel Prize in Chemistry in 2001, Noyori shared a half of the prize with William S. Knowles for the study of chirally catalyzed hydrogenations; the second half of the prize went to K. Barry Sharpless for his study in chirally catalyzed oxidation reactions ( Sharpless epoxidation). Education and career Ryōji Noyori was born in Kobe, Japan. Early in his school days Ryoji was interested in physics. His interest was kindled by the famous physicist Hideki Yukawa (1949 Nobel Prize in Physics winner), a close friend of his father. Later, he became fascinated with chemistry, after hearing a presentation on nylon at an industrial exposition. He saw the power of chemistry as being the ability to "produce high value from almost nothing". He was a student at the School of Engineering (Department of Industrial Chemistry) of the Kyoto University, where he graduated in 1961. He subsequently obtained a Master's degree in Industrial Chemistry from the Grad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corey–Fuchs Reaction
The Corey–Fuchs reaction, also known as the Ramirez–Corey–Fuchs reaction, is a series of chemical reactions designed to transform an aldehyde into an alkyne. The formation of the 1,1-dibromoolefins via phosphine-dibromomethylenes was originally discovered by Desai, McKelvie and Ramirez. The phosphine can be partially substituted by zinc dust, which can improve yields and simplify product separation. The second step of the reaction to convert dibromoolefins to alkynes is known as Fritsch–Buttenberg–Wiechell rearrangement. The overall combined transformation of an aldehyde to an alkyne by this method is named after its developers, American chemists Elias James Corey and Philip L. Fuchs. By suitable choice of base, it is often possible to stop the reaction at the 1-bromoalkyne, a useful functional group for further transformation. Reaction mechanism The Corey–Fuchs reaction is based on a special case of the Wittig reaction, where two equivalents of triphenylphosphine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Johnson–Corey–Chaykovsky Reaction
The Johnson–Corey–Chaykovsky reaction (sometimes referred to as the Corey–Chaykovsky reaction or CCR) is a chemical reaction used in organic chemistry for the synthesis of epoxides, aziridines, and cyclopropanes. It was discovered in 1961 by A. William Johnson and developed significantly by E. J. Corey and Michael Chaykovsky. The reaction involves addition of a sulfur ylide to a ketone, aldehyde, imine, or enone to produce the corresponding 3-membered ring. The reaction is diastereoselective favoring ''trans'' substitution in the product regardless of the initial stereochemistry. The synthesis of epoxides via this method serves as an important retrosynthetic alternative to the traditional epoxidation reactions of olefins. The reaction is most often employed for epoxidation via methylene transfer, and to this end has been used in several notable total syntheses (See Synthesis of epoxides below). Additionally detailed below are the history, mechanism, scope, and enanti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CBS Catalyst
The CBS catalyst or Corey–Bakshi–Shibata catalyst is an asymmetric catalyst derived from proline. It finds many uses in organic reactions such as the CBS reduction, Diels-Alder reactions and (3+2) cycloadditions. Proline, a naturally occurring chiral compound, is readily and cheaply available. It transfers its stereocenter to the catalyst which in turn is able to drive an organic reaction selectively to one of two possible enantiomers. This selectivity is due to steric strain in the transition state that develops for one enantiomer but not for the other. Synthesis The CBS catalyst can be prepared from diphenylprolinol, condensed with a phenylboronic acid, or with borane (as shown below). The CBS catalyst then complexes ''in situ'' with borane to give the active catalyst. Use The general outline for the organic synthesis of a CBS catalyst is shown below. The first leg of the reaction sequence starts from the azeotropic dehydration of a boronic acid (1) such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthon
In retrosynthetic analysis, a synthon is a hypothetical unit within a target molecule that represents a potential starting reagent in the retroactive synthesis of that target molecule. The term was coined in 1967 by E. J. Corey. He noted in 1988 that the "word ''synthon'' has now come to be used to mean synthetic ''building block'' rather than retrosynthetic fragmentation structures". It was noted in 1998 that the phrase did not feature very prominently in Corey's 1981 book ''The Logic of Chemical Synthesis'', as it was not included in the index. Because synthons are Ion, charged, when placed into a synthesis an uncharged form is found commercially instead of forming and using the potentially very unstable charged synthons. Example : In planning the synthesis of phenylacetic acid, two synthons are identified: a nucleophilic "COOH−" group, and an electrophilic "PhCH2+" group. Of course, both synthons do not exist by themselves; synthetic equivalents corresponding to the synthons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retrosynthetic Analysis
Retrosynthetic analysis is a technique for solving problems in the planning of organic syntheses. This is achieved by transforming a target molecule into simpler precursor structures regardless of any potential reactivity/interaction with reagents. Each precursor material is examined using the same method. This procedure is repeated until simple or commercially available structures are reached. These simpler/commercially available compounds can be used to form a synthesis of the target molecule. Retrosynthetic analysis was used as early as 1917 in Robinson's Tropinone total synthesis. Important conceptual work on retrosynthetic analysis was published by George Vladutz in 1963. E.J. Corey formalized and popularized the concept from 1967 onwards in his article ''General methods for the construction of complex molecules'' and his book ''The Logic of Chemical Synthesis''. The power of retrosynthetic analysis becomes evident in the design of a synthesis. The goal of retrosynthetic a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jin-Quan Yu
Jin-Quan Yu () is a Chinese-born American chemist. He is the Frank and Bertha Hupp Professor of Chemistry at Scripps Research, where he also holds the Bristol Myers Squibb Endowed Chair in Chemistry. He is a 2016 recipient of the MacArthur Fellowship, and is a member of the American Academy of Arts and Sciences, American Association for the Advancement of Science, and the Royal Society of Chemistry. Yu is a leader in the development of C–H bond activation reactions in organic chemistry, and has reported many C–H activation reactions that could be applicable towards the synthesis of drug molecules and other biologically active compounds. He also co-founded Vividion Therapeutics in 2016 with fellow Scripps chemists Benjamin Cravatt and Phil Baran, and is a member of the scientific advisory board of Chemveda Life Sciences. Early life and education Yu was born on January 10, 1966, in Zhejiang, China. He received his B.Sc. in chemistry at East China Normal University in 1987. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ramakanth Sarabu
Ramakanth Sarabu (June 20, 1955 – February 11, 2021) was an Indian organic chemist. He is known for his contributions in diabetes research, specifically the work of Glucokinase activation as a treatment therapy for type 2 diabetes. Biography Ramakanth Sarabu was born in Hyderabad, Telangana, where he received his early education. He earned a master's degree from Osmania University in organic chemistry and his doctorate from Indian Institute of Technology, Madras in molecular rearrangements from 1979 to 1984. In 1984 he moved to the US to pursue post-doctoral fellowship under Elias James Corey, at Harvard University. He did a second post-doctoral fellowship in 1985 at Case Western University in Cleveland, OH. He is known for his works and contributions in the domain of Glucokinase activation as a treatment therapy for type 2 diabetes. He died on 11 February 2021 in Montville, New Jersey New Jersey is a U.S. state, state located in both the Mid-Atlantic States, Mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |